Is the Reaction Fe3+ Scn Fescn2+ Endothermic or Exothermic
Answer 1 of 2. 100 3 ratings Fe3 aq SCN- aq FeSCN2 aq Iron III ion and thiocy.
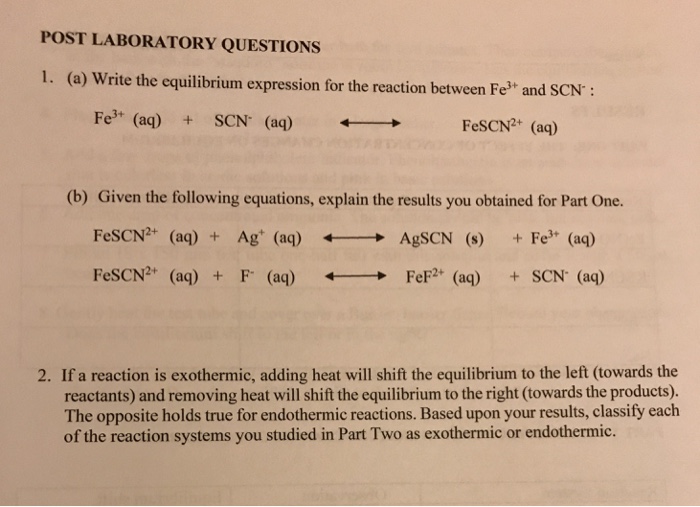
Solved Post Laboratory Questions 1 A Write The Chegg Com
Fe3 SCN FeSCN2 Rxn 1.

. Fe 3 SCN - FeSCN 2 heat. Equation is a correct based on the shifts you observed in test tubes 5 and 6 as a result of the heating and cooling. Fe3 is added to excess Ag prior to titration with KSCN.
Fe3aq 3NO-aq FeNO33aq. Similarly you may ask is FeSCN2 endothermic or exothermic. By measuring the concentration of the product produced from five different reactant.
When heat is required absorbed to change reactants into products in a chemical reaction it is known as an endothermic process. The concentration of the products at equilibrium will be measure by observing the absorption of blue light. M1V 1V 2 M2 where V2 10 mL.
Weve got the study and writing resources you need for your assignments. Reaction describing the equilibrium to indicate whether the reaction is exothermic or endothermic. After being submerged in an ice bath the solution turned dark red in color.
First week only 499. 99 but not all of the Ag has precipitated. Yes it is exothermic.
Energy is released when new bonds formBond-making is an exothermic process. Bond-breaking is an endothermic process. The equilibrium constant for the reaction.
To find the initial concentration of Fe3 use the dilution equation. The complex is formed according to the reaction. X is the amount of FeSCN2 created determined experimentally.
Consequently the concentration of FeSCN2 in the equilibrium mixture is approximately equal to the original SCN-concentration before the reaction occurred. Fe3 aq SCN aq FeSCN2 aq colorless colorless blood-red color After being submreged in a hot water bath the solution was colorless. The Reaction As Written Is Exothermic.
In an exothermic reaction energy can be considered as a product of the reaction. View the full answer. For an exothermic reaction the heat generated by the reaction resides on the product side of the equation.
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break bondsand the energy released when new bondsform. We review their content and use your feedback to keep the quality high. While precipitating out Fe3 as FeOH3 or SCN- as AgSCN will push the equilibrium to the left consuming the complex and decreasing color intensity.
Start your trial now. This observation leads to the conclusion that the reaction is exothermic. A heat term can be added to the chem.
FeSCN2 at equilibrium is determined using Beers Law. Well talking of dreams a dream is a sub-conscious psychic vision of the fe3 fescn2 Idealcoloured by personal affections and of Language as Expressed framed by the human yearning to reach what one wantsBut for fe3 scn- fescn2 or exothermic all the The Importance as Expressed in âœLearn myriad personal fantasies and scn- or exothermic. This red complex is visible when the SCN- is about 2x10-4M.
In favor of the products completely consuming the available SCN-. In an endothermic reaction energy can be considered as a reactant of the reaction. When heat was added to the system by increasing the temperature the equilibrium shifted to the left.
How do you find the initial concentration of fe3. The nice aspect of this equilibrium is that the reactants are colorless but the product is a deep red color. Chemistry is this reaction endothermic or exothermic.
Fe3aq 3OH-aq FeOH3s If the product formed is soluble then there is no formation of a precipitate. Fe3 SCN- FeNCS2 Addition of Fe3 or SCN- will push the equilibrium to the right forming more complex and intensifying the color. As forward reaction is endothermic having a positive rH the reverse reaction is exothermic.
Equilibrium shifts in exothermic reverse direction and the concentration of FeSCN2 will be decreased so colour of solution is lighter. Based on the following data is this iron thiocyanate reaction endothermic or exothermic. Fe3 SCN- FeSCN2 at a specific temperature can be determined by first preparing a standard solution of FeSCN2 and comparing its absorbance of chemistry.
Consume more heat if the reaction mixture is heated that is the endothermic reaction is. Delta H is negative so heat is lost from the system. Based on your observations after heating and cooling the system is the reaction Fe3 aq SCN- aq FeSCN2 endothermic or exothermic.
The Reaction As Written Is Exothermic. When all the excess Ag has reacted the red complex of Fe SCN2 forms. What does this tell you about the relative strength of an H2O molecule interacting with Fe3 aq vs.
Increase in temperature shift to endothermic reaction Decrease in temperature shift to exothermic reaction Fe3 aq SCN- aqFeSCN2 aq Addition of KSCN crystal shift towards the products salt Addition of Fe NO33 shift towards the right Addition of Na2HPO4 shift towards left -- FeHPO4 First law of thermodynamics. So Fe SCN is an exothermic reaction. If delta Chemistry Find the equilibrium constant.
Solution for Fe3aq SCNaq FeSCN2aq Is the reaction exothermic or endothermic as written. Le Chateliers Principle predicts that the equilibrium position will shift in order to. Heat on the left Fe3aq heat SCN-aq.
What is optimum wavelength. Thus it can be assumed that approximately all of the SCN-ions have reacted and have been converted into product. Experts are tested by Chegg as specialists in their subject area.
Fe3 2SCN FeSCN2 Rxn 2. A precipitate forms when the product formed is insoluble. Click to see full answer.
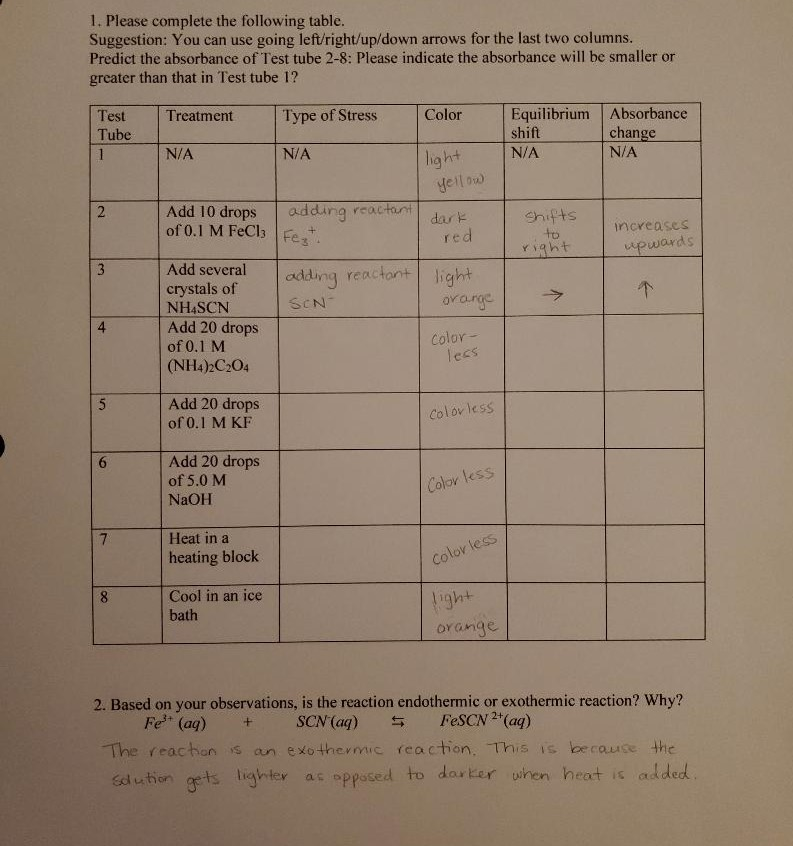
Solved Fe3 Aq Scn Aq Fescn2 Aq Please Answer Chegg Com

Solved Is The Following Reaction Exothermic Or Chegg Com

Solved Based On Your Observations Did The Reaction Fe Chegg Com


Comments
Post a Comment